News
Page: 7
ARBOR VITAE – The Arbor Vitae Fire Department spent this past Saturday on Musky Mountain, working on low angle rescues. The Tech rescue team trains for all variety of emergency situations, including low angle training, which takes 40 hours to complete the course. Rescues like a car driving down a steep slope are what low […]
MADISON, Wis. (AP) — Wisconsin wildlife officials estimate the number of elk in the state will grow to about 500 animals by July. The Department of Natural Resources began reintroducing elk to the state in 1995 by importing 25 animals to the Clam Lake region. The agency began another reintroduction effort in 2014 that called […]
ROYAL OAK, Mich. (AP) — Michigan Gov. Gretchen Whitmer gave final approval Monday to the state’s new red flag law during a bill signing just outside of Detroit. The law is expected to take effect next spring and will allow family members, police, mental health professionals, roommates and former dating partners to petition a judge […]
GOGEBIC COUNTY – FEMA Coming to Gogebic and Ontonagon Counties Soon to Inspect Flood Damage in the U.P. Officials from FEMA will soon be in the U.P. to inspect damage in seven counties, including Alger, Baraga, Gogebic, Houghton, Iron, Marquette and Ontonagon. Agency representatives will be reviewing the condition of public infrastructure only. Assistance for […]
IRONWOOD – Gas prices in Michigan are up 23 cents from a week ago. Michigan drivers are now paying an average of $3.57 per gallon for regular unleaded. This price is 8 cents less than this time last month and a $1 less than this time last year. Michigan motorists are seeing a sharp spike […]
LANSING, Mich. (AP) — Michigan’s revenue is expected to be nearly $900 million less this fiscal year than had been previously forecasted in January due to an income tax rate reduction and corporate tax incentives, state officials said Friday. The lowered projections could complicate the state’s next budget, which lawmakers are currently working to finish […]
ONTONAGON – Ontonagon Lake Trout Classic Wraps Up. This year’s 20th Annual Ontonagon Lake Trout Classic wrapped yesterday, with anglers aiming to pull in the biggest trout they could, weighing in their six heaviest, in hopes of catching the heaviest fish weight. Over the last 20 years, the Classic has raised more than a hundred […]
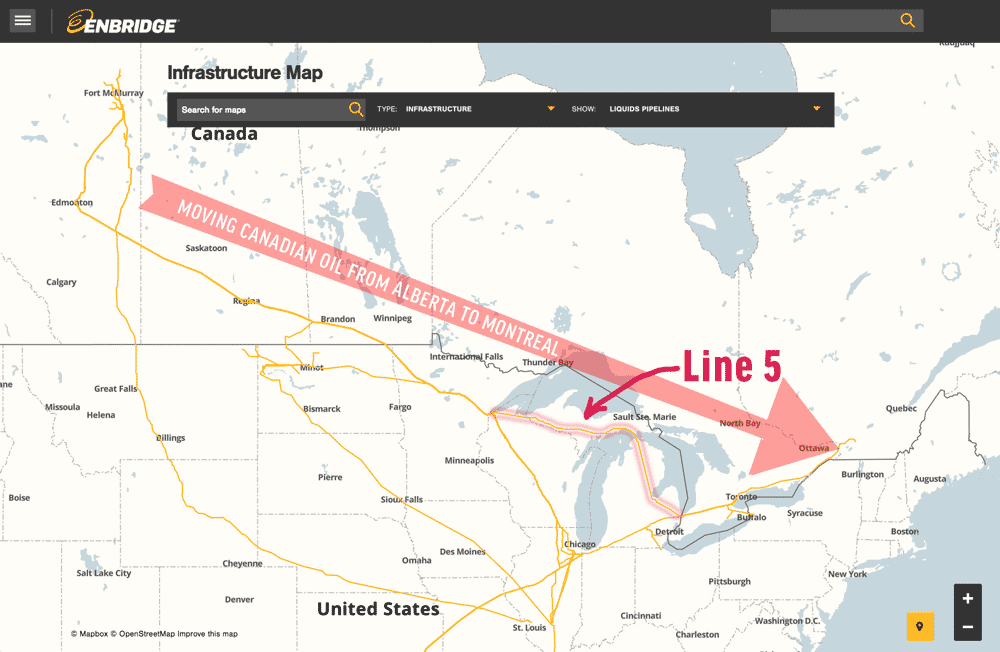
ODANAH (AP) — A federal judge signaled Thursday that he won’t order an energy company to shut down an oil pipeline. The Bad River tribe argues that rapid erosion could expose the line and cause a massive oil spill on reservation land in northern Ashland County. In some places, the Bad River now flows less […]
MERCER – The Iron County Planning Committee Says No to a Campground being Built in the Mercer Area. An application to build a campground with 3 rental cabins, storage buildings and pontoon and snowmobile rentals has been denied by the Iron County Comprehensive Planning/Land and Zoning Committee the committee met recently to consider the application […]
UPPER MICHIGAN – Results of 5 County Survey on Mental Health Show a Healthier Attitude. The results of a survey of almost 900 Baraga, Gogebic, Houghton, Keweenaw and Ontonagon County residents asking questions regarding mental health and substance use issues was discussed during a public meeting on mental health and wellness at the Portage Lake […]